62nd National Congress of the Italian Society of Rheumatology
Vol. 77 No. s1 (2025): Abstract book of the 62th Conference of the Italian Society for Rheumatology, Rimini, 26-29 November 2025
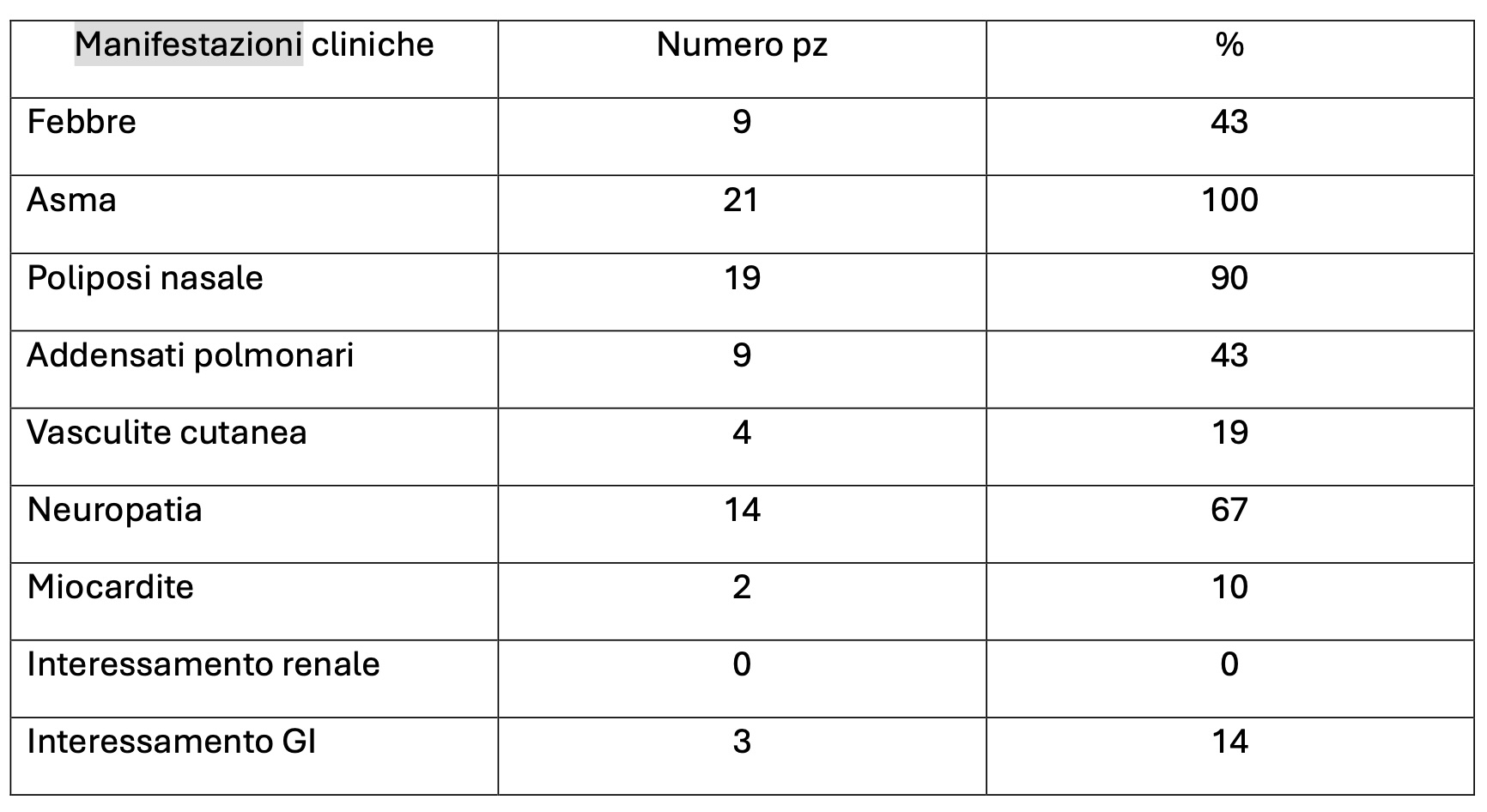
PO:37:260 | Mepolizumab in the treatment of patients with eosinophilic granulomatosis with polyangiitis: real-life experience from a single-center cohort
Eleonora Bruschi1, Fabrizio Angeli1, Alessandro Belotti Masserini1, Stefania Bertocchi1, Silvia Breda1, Elide Lupi1, Valeria Rossi1, Tania Ubiali1, Massimiliano Limonta1 | 1ASST Papa Giovanni XXIII Bergamo Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 26 November 2025
109
Views
0
Downloads