62nd National Congress of the Italian Society of Rheumatology
Vol. 77 No. s1 (2025): Abstract book of the 62th Conference of the Italian Society for Rheumatology, Rimini, 26-29 November 2025
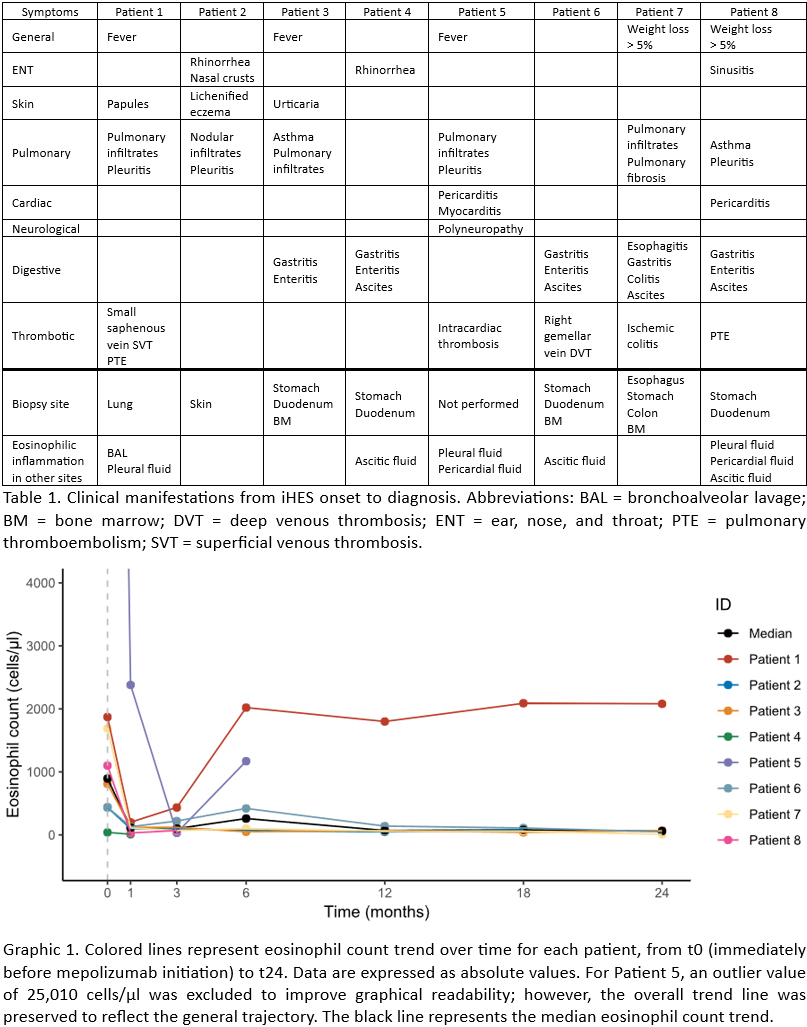
PO:22:029 | Impact of mepolizumab on idiopathic hypereosinophilic syndromes: analysis of a monocentric retrospective cohort
Jacopo Mora1, Francesca Regola1, Paola Toniati2, Ilaria Cavazzana1, Franco Franceschini1. | 1ASST Spedali Civili e Università degli Studi di Brescia, Brescia, Italy; 2ASST Spedali Civili di Brescia, Brescia, Italy.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 26 November 2025
94
Views
0
Downloads




 References: (1) Valent P, et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy. 2023 Jan;78(1):47-59
References: (1) Valent P, et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy. 2023 Jan;78(1):47-59