62nd National Congress of the Italian Society of Rheumatology
Vol. 77 No. s1 (2025): Abstract book of the 62th Conference of the Italian Society for Rheumatology, Rimini, 26-29 November 2025
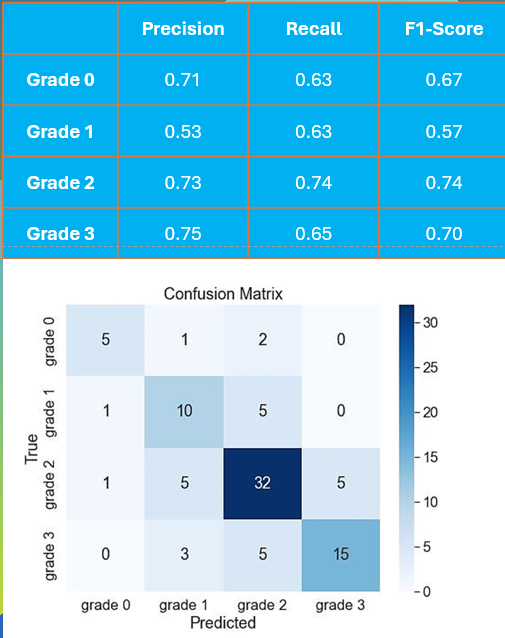
CO:05:2 | An OMERACT study for the development of an algorithm for automatic identification of calcium pyrophosphate deposition by ultrasound: the Crystal Artificial Intelligence Monitoring Study
Daniele Cirillo1, Tito Bassani2, Silvia Sirotti1|2, Greta Pellegrino1|2, Alessandro Lucia1, Rodolfo Fabbri1, Laura Pezzoni1, Piercarlo Sarzi Puttini1|2, Georgios Filippou1|2. | 1Università degli Studi di Milano; 2IRCCS Ospedale Galeazzi - Sant'Ambrogio, Milano, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 26 November 2025
110
Views
0
Downloads