62nd National Congress of the Italian Society of Rheumatology
Vol. 77 No. s1 (2025): Abstract book of the 62th Conference of the Italian Society for Rheumatology, Rimini, 26-29 November 2025
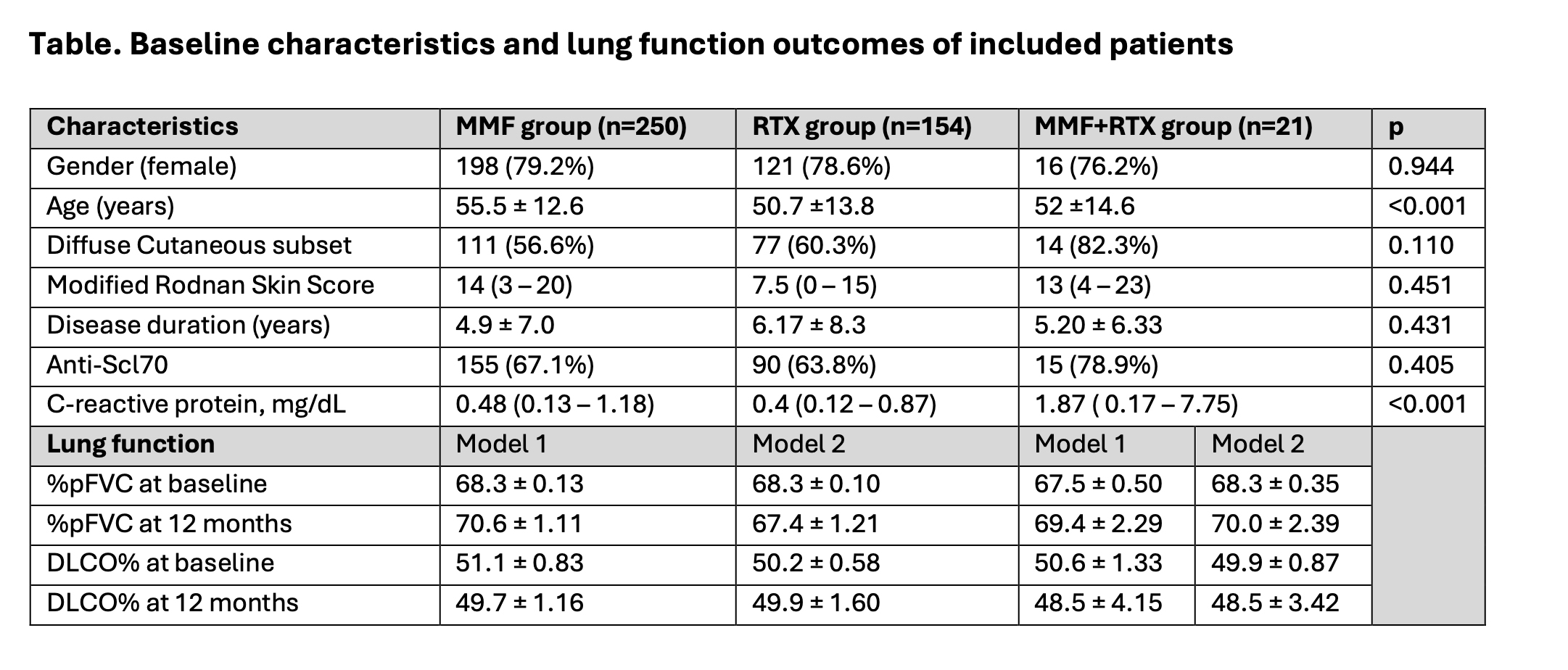
PO:32:176 | Outcomes of upfront combination vs monotherapy with rituximab or mycophenolate mofetil for systemic sclerosis interstitial lung disease: results from a European Scleroderma Trials and Research cohort study
Devis Benfaremo1, Corrado Campochiaro2, Gábor Kumánovics3, Christina Bergmann4, Elisabetta Zanatta5, David Launay6, Serena Guiducci7, Mickael Martin8, Carolina De Souza Müller9, Carlomaurizio Montecucco10, Luc Mouthon11, Gabriella Szucs12, Kastriot Kastrati13, Marie-Elise Truchetet14, Madelon Vonk15, Francesco Del Galdo16, Marco Matucci-Cerinic2, Gianluca Moroncini1, Yannick Allanore17. | 1Department of Clinical and Molecular Sciences, Marche Polytechnic University, Marche University Hospital, Ancona, Italy; 2Vita-Salute San Raffaele University, San Raffaele Hospital, Milano, Italy; 3University of Pécs, Department Of Rheumatology And Immunology, Pécs, Hungary; 4University Hospital Erlangen, Department Internal Medicine 3, Erlangen, Germany; 5Padova University Hospital, Rheumatology Unit, Padova, Italy; 6Univ. Lille, Inserm, CHU Lille, Department of Internal Medicine and Clinical Immunology, Lille, France; 7University of Florence, Azienda Ospedaliera Universitaria Careggi, Firenze, Italy; 8Poitiers University Hospital, Department of Internal Medicine Poitiers France; 9Hospital de Clinicas da Universidade Federal do Parana Curitiba Brazil; 10Università di Pavia e IRCCS Fondazione Policlinico S. Matteo, Pavia, Italy; 11Hôpital Cochin, Department of Internal Medicine, Paris, France; 12University of Debrecen, Faculty of Medicine, Department of Rheumatology, Debrecen, Hungary; 13Medical University of Vienna, Department of Medicine III, Division of Rheumatology, Vienna, Austria; 14CHU de Bordeaux, Rheumatology department, Bordeaux, France; 15Radboudumc, Department of Rheumatology Nijmegen The Netherlands; 16Leeds Raynauds and Scleroderma Program, NIHR Biomedical Research Centre, Leeds, United Kingdom; 17Université Paris Cité, Cochin Hospital, Rheumatology Department, Paris, France.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 26 November 2025
76
Views
0
Downloads
Devis Benfaremo, Corrado Campochiaro, Gábor Kumánovics, Christina Bergmann, Elisabetta Zanatta, David Launay, Serena Guiducci, Mickael Martin, Carolina De Souza Müller, Carlomaurizio Montecucco, Luc Mouthon, Gabriella Szucs, Kastriot Kastrati, Marie-Elise Truchetet, Madelon Vonk, Francesco Del Galdo, Marco Matucci-Cerinic, Gianluca Moroncini, Yannick Allanore