62nd National Congress of the Italian Society of Rheumatology
Vol. 77 No. s1 (2025): Abstract book of the 62th Conference of the Italian Society for Rheumatology, Rimini, 26-29 November 2025
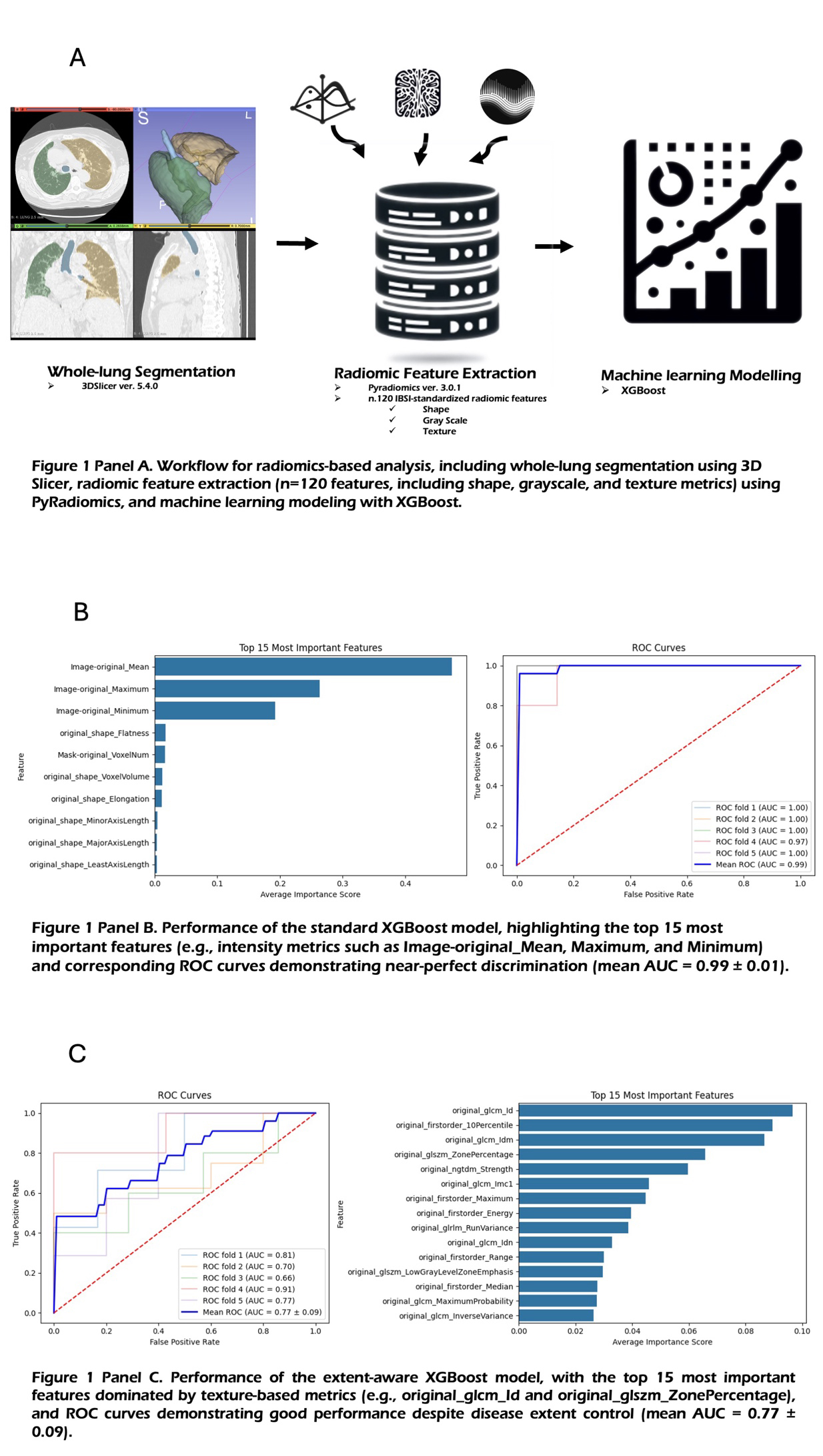
CO:05:3 | Radiomics and machine learning for differentiating rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis with usual interstitial pneumonia pattern
Vincenzo Venerito1, Chiara Nani2, Andreina Manfredi3, Marco Fornaro1, Giuseppe Lopalco1, Florenzo Iannone1, Cecilia Burattini2, Marco Sebastiani4. | 1Rheumatology Unit, University of Bari; 2Respiratory Unit, Guglielmo da Saliceto Civil Hospital, Piacenza; 3Rheumatology Unit, Azienda Unità Sanitaria Locale-IRCCS di Reggio Emilia; 4Rheumatology Unit, AUSL Piacenza, University of Parma, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 26 November 2025
117
Views
0
Downloads