Development of thymoma without myasthenia gravis in a patient with radiographic axial spondyloarthritis treated with tumor necrosis factor-α inhibitors
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accepted: 18 September 2024
Authors
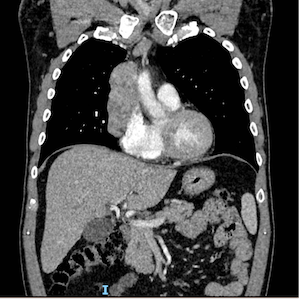
Thymic tumors are rare in the general population, and to the best of our knowledge, no cases of thymoma have been described in patients with rheumatic diseases treated with tumor necrosis factor (TNF)-α inhibitors, except for the case of a patient receiving infliximab for Crohn’s disease (CD) who developed a B2 thymoma. We describe a 60-year-old Caucasian male with radiographic axial spondyloarthritis (r-axSpA) and CD who developed an AB-type thymoma without myasthenia gravis after 18 years of treatment with TNF-α inhibitors. The patient had received the same molecule since the r-axSpA/CD diagnosis and changed it 6 months before the diagnosis of thymoma due to a disease flare. At the time of the drug switch, no mediastinal mass was present on the chest X-ray. The thymoma was surgically removed, and no additional therapy was needed. Treatment with TNF-α inhibitors was reintroduced after surgery. This case raises some important questions that remain open and deserve to be addressed in the future, such as the association between immunosuppressive therapy and thymoma and the controversial relationship between TNF-α inhibitors and myasthenia gravis.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.











