POSTER SESSION 1: PSORIATIC ARTHRITIS AND SERONEGATIVE SPONDYLOARTHRITIS (I)
9 October 2025
2025: Prova Issue
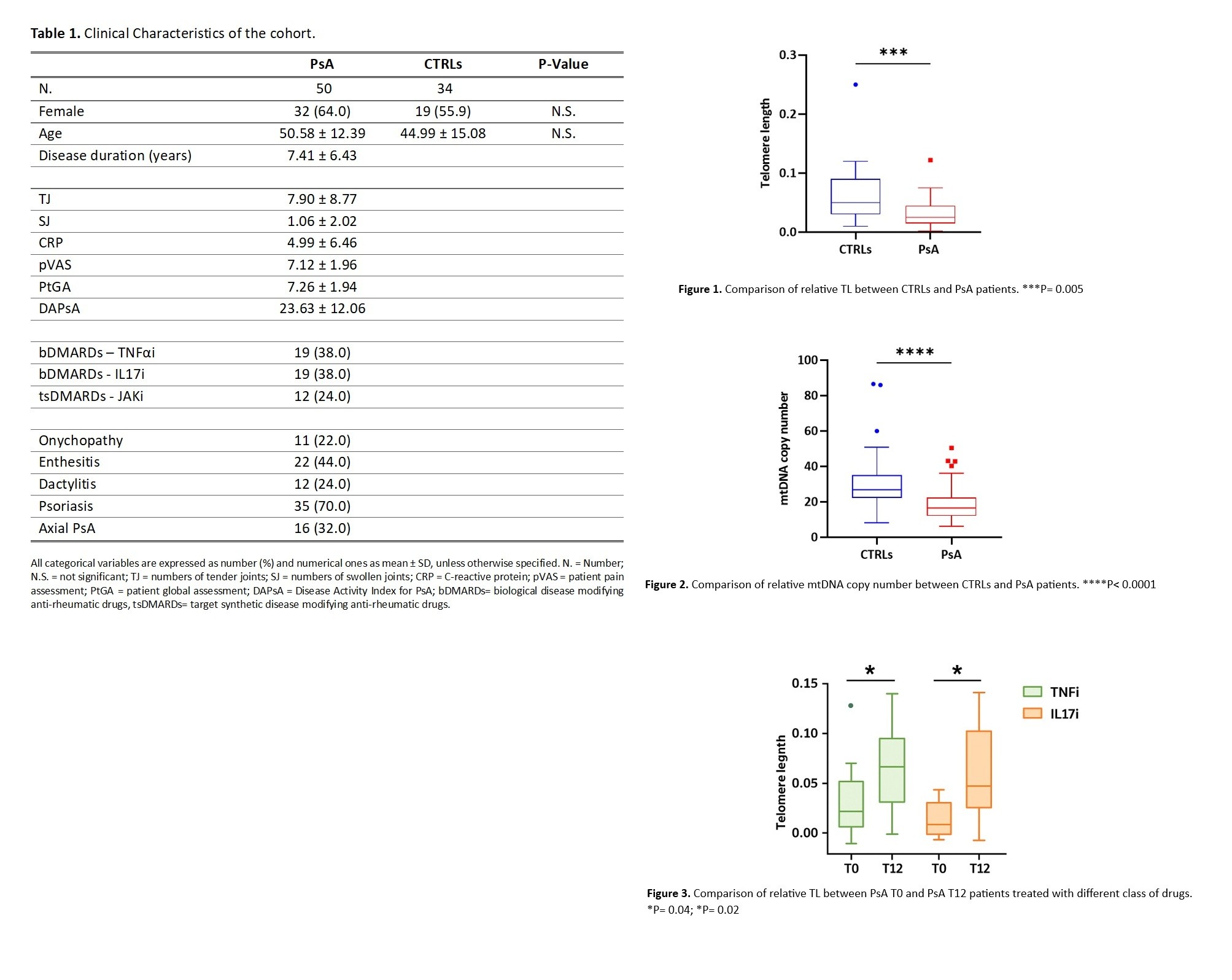
PO:01:007 | Modulation of cellular senescence in psoriatic arthritis: exploring the potential impact of b/tsdmards treatment on telomere length, mtdna copy number, and oxidative damage
Eneida Cela1, Giada De Benedittis2, Arianna D'Antonio1, Andrea Latini3, Chiara Morgante2, Cinzia Cicacci3, Paola Conigliaro1, Mauro Fatica1, Giulia Mori1, Giuseppe Novelli2, Paola Borgiani2, Maria Sole Chimenti1. | 1Rheumatology, Allergology and Clinical Immunology, Department of System Medicine, University of Rome Tor Vergata Rome; 2Department of Biomedicine and Prevention, Section of Genetics, University of Rome Tor Vergata, Rome; 3UniCamillus Saint Camillus International University of Health Sciences, Rome, Italy.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
10
Views
0
Downloads