Vasculitis associated with adenosine deaminase 2 deficiency: at the crossroads between Behçet’s disease and autoinflammation. A viewpoint
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
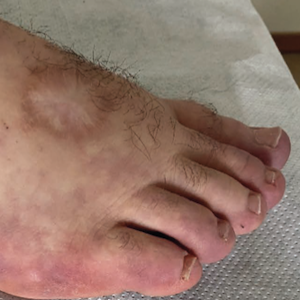
Adenosine deaminase 2 deficiency (DADA2) is a rare monogenic vasculopathy caused by loss-of-function homozygous or compound heterozygous mutations in ADA2, formerly CECR1 (cat eye syndrome chromosome region 1) gene. The DADA2 phenotype is widely heterogeneous, and patients may present with fever, weight loss, livedo reticularis/racemosa, digital ischemia, cutaneous ulceration, peripheral neuropathy, abdominal pain, bowel perforation, and portal or nephrogenic hypertension. More specific manifestations include early-onset ischemic or hemorrhagic stroke, mild immunodeficiency and hypogammaglobinemia, cytopenia, and vision disturbances. Herein, we present the case of a young male with vasculitis associated with DADA2. The presence of HLA-B51 and the clinical features of this patient raised the question of similarities between ADA2 deficiency, Behçet’s disease, and NOD2-associated diseases. Treatment of this rare monogenic disease is challenging and based on small case series. The long-term experience of this patient proved the difficulties of prednisone tapering and the lack of satisfactory therapeutic strategies.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.